L. Tamás, T. E. Gunda, Gy. Batta, F. Sztaricskai:
On the Preparation of 2-Substituted Cephalosporins. Part 2. Diels-Alder and 1,3-Dipolar Cycloadditions of 2-Crotonoyl-, 2-Sorbyl- and 2-Cinnamoyl-deacetoxycephalosporanate 1β-Oxides.
Helv. Chim. Acta, 86, 50-58 (2003)
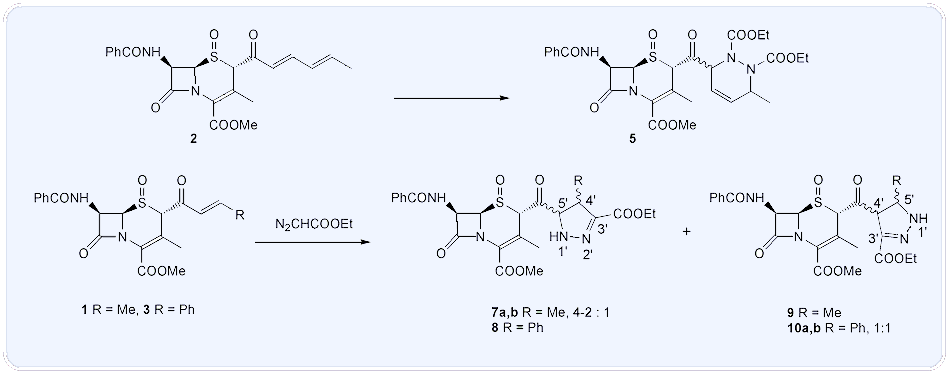
Abstract: Cephalosporin sulfoxides 1, 2 and 3 containing α,β-enone- or dienone-type moieties at position 2 were reacted with 2,3-dimethylbutadien or diethyl azodicarboxylate in Diels-Alder reactions to synthesize new cephalosporin derivatives 4 and 5 with a heterocyclic substituent. Under the same conditions ethyl diazoacetate and diazomethane reacted differently: while reactions with the former lead to compounds 12-15 corresponding to the 1,3-dipolar cycloaddition route, diazomethan produced only enolethers. This difference could be rationalized by assuming two different reaction pathways: an orbital symmetry controlled concerted cycloaddition and an ionic one.