László Tamás, Gabriella N. Szőke, Tamás E. Gunda and Ferenc Sztaricskai:
On the Anomalous N-Methyl-morpholine N-oxide/Osmium Tetroxide Oxidation of Penicillins and Cephalosporins.
ACH - Modells in Chemistry, 135, 207 (1998).
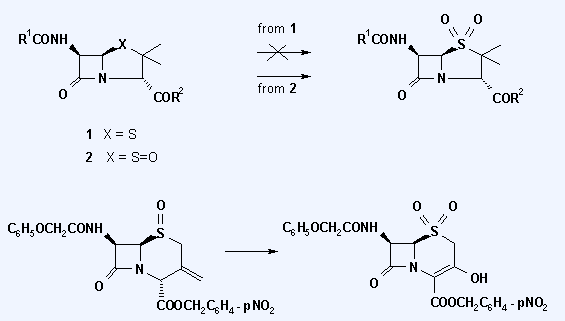
Abstract: The penicillin and cephalosporin sulfides and β-sulfoxides behave suprisingly disparately when subjected to oxidation with N-methyl-morpholine N-oxide and osmium tetroxide: while penicillins are inert to this oxidation procedure, their sulfoxides are cleanly converted to the sulfones. In the case of cephalosporins only Δ3 → Δ2 isomerization was observed. Cephem sulfoxides failed to react or yielded only degradation products, only the 3-exomethylene-cepham derivative yielded the corresponding 3-hydroxy-cephem sulfone.